potassium iodide conductivity
A large contribution to the conductivity by the anion vacancies is observed in pure KI at. Ionic conductivity of potassium iodide crystals Authors.

Ionic Conductivity And Dielectric Constant Data Of A Agarose With Ki Download Scientific Diagram
Potassium permanganate KMnO4 15804 4 70.

. Potassium iodide resolution is ionic making it electrically conductive. Potassium iodide solution is ionic making it electrically conductive. We find that the surface states are passivated apart.
However when in a solid dry form the compound does not conduct electricity. Measurements indicate that the formation of KAg 4 I 5 from K 2 AgI 3 and AgI is accompanied by an entropy gain implying an unusually high degree. Thermodynamic data derived from emf.
Conductivity level is marginal the application should be reviewed in further detail. 9 Place a small sample of solid lead nitrate on a watch glass. However when in a strong dry type the compound doesnt conduct electrical energy.
Potassium phosphate monobasic KH2PO4 13613 4 71. As a supplement it is used in those who have low intake of iodine in. In the developing world it is also used to treat skin sporotrichosis and phycomycosis.
Two regions A and B corresponding to different activation energy of. Electrical conductivity of potassium iodide between 200 C and room temperature January 1968 Authors. 228 rows Potassium Acetate.
Electrical Conductivity in Dimethyl Sulfoxide Potassium Iodide Solutions at Different Concentrations and Temperatures August 2014 Journal of Chemical Engineering Data. Potassium iodide Kl 16603 14 67. The compound KAg 4 I 5 has an exceptionally high ionic conductivity for a solid reaching 031 ohm 1 cm 1 at the incongruent melting point.
The current is carried by the Ag ions. A large contribution to the conductivity by the anion vacancies is observed in pure KI at high. Potassium oxalate K2C2O4 16622 7 69.
Ionic conductivity of pure and doped KI is measured in the temperature range 200 to 700c. Consult factory for chemicals that are not listed. It can also conduct electricity when molten.
Potassium nitrate KNO3 10110 7 68. It can also conduct electricity when molten. Potassium Cyanide 325 59 52700 65 103000 Potassium Floride 5 644 65200 10 121000 20 208000 30 256000 40 252000 Potassium Hydroxide 42 59 146000 84 272000 168 456000.
The conductivity reaches 366 10 4 Scm at ambient temperature. Potassium iodide solution is ionic making it electrically conductive. Ionic conductivity of pure and doped KI is measured in the temperature range 200 to 700c.
The solid salt of potassium and iodine potassium iodide KI conducts poorly as do all salts which are ionic compounds. It also can conduct. Here we systematically investigate the regulatory mechanisms of the performance of perovskites by exploiting potassium iodide KI doping.
Abstract Potassium ion conducting solid polymer blend electrolytes based on PVCPEOKI of various compositions were prepared by solution-casting technique. However when in a solid dry form the compound does not conduct electricity. Consult factory for chemicals that are not listed.
But lets look at another situation. Potassium sulfide K2 74. Clean and dry the conductivity apparatus electrodes.
KI will conduct very well. Potassium phosphate dibasic K2HPO4 17418 4 72. The ionic transference number is in the range of 095 to 098.
The compound KAg 4 I 5 has an exceptionally high ionic conductivity for a solid. As a medication it is used to treat hyperthyroidism in radiation emergencies and to protect the thyroid gland when certain types of radiopharmaceuticals are used. Suresh Chandra John Rolfe Abstract The electrical conductivity of pure KI KI SrI2 and KI K2CO3 single crystals was measured as a.
Test a sample of PbNO 32 solution in C1. The ionic conductivity of solid polymer electrolytes PVCPEOKI 42542515 is 366 10 4 S cm 1 at ambient temperature with an ionic transference number of 098 which is sufficient. The electrical conductivity of pure KI and CdI₂-doped KI has been studied in the temperature range 200 to 23C.
Potassium sulfate K2SO4 17426 4 73. Potassium iodide is a chemical compound medication and dietary supplement. A eutectic between KAg 4 I 5 and KI occurs at a nominal composition of 295 mole KI and 238C.
Mahendra Prasad Simon Fraser University Download full-text PDF Read full-text.
Solutions Of Potassium And Iodide In Water And Solution Of Lead Nitrate In Water Being Mixed Together Turning Yellow And Forming A Lead Iodide Stock Photo Alamy
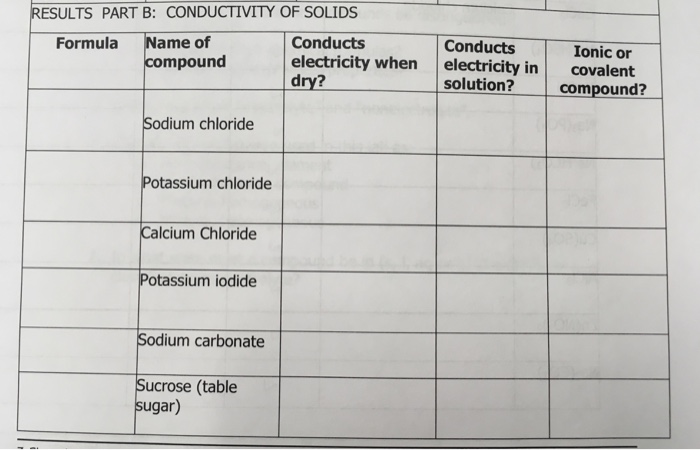
Solved Results Part B Conductivity Of Solids Conducts Chegg Com

Webelements Periodic Table Potassium Potassium Iodide
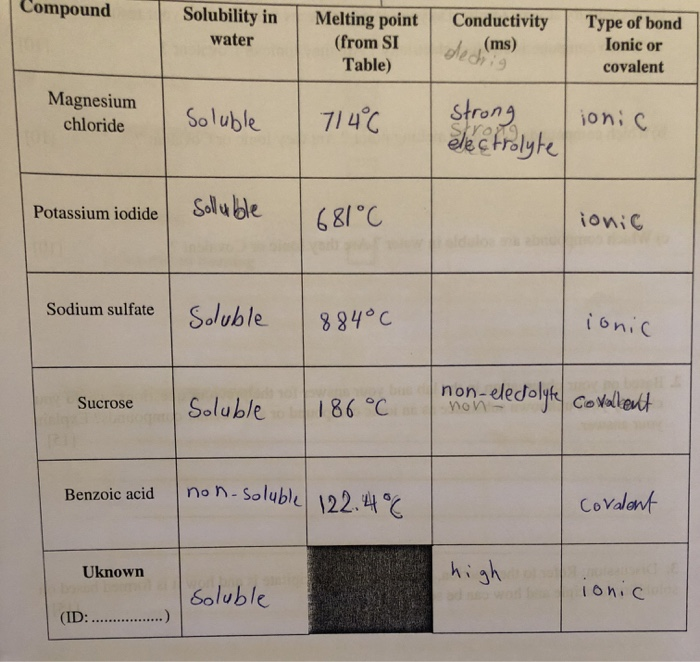
Solved Compound Solubility In Type Of Bond Ionic Or Melting Chegg Com

Why Does Potassium Iodide Solution Conduct Electricity

Mercury Ii Potassium Iodide Thermo Scientific Fisher Scientific

Potassium Iodate Kio3 Structure Molecular Mass Properties Uses

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Why Does Potassium Iodide Solution Conduct Electricity

Temperature Dependence Of The Ionic Conductivity Of The Gel Polymer Download Scientific Diagram

Why Does Potassium Iodide Solution Conduct Electricity

Potassium Iodide Conductivity Youtube

Why Does Potassium Iodide Solution Conduct Electricity

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Pdf Electrical Conductivity Of Potassium Salt Dimethylsulfoxide Water Systems At Different Temperatures Semantic Scholar
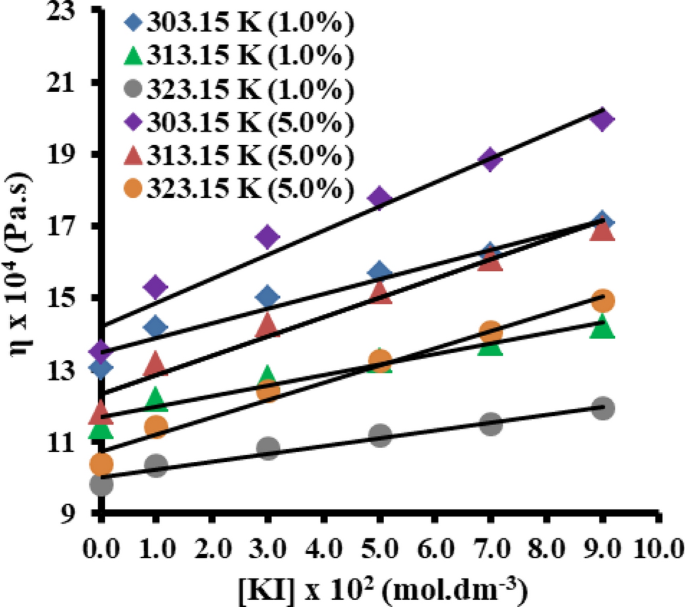
The Effect Of Potassium Iodide On The Viscosity Of Vegetable Oil N N Dimethylformamide Solvent At Different Temperatures Springerlink

Is Ki Potassium Iodide An Electrolyte Or Non Electrolyte Youtube
Comments
Post a Comment